News & Announcements
Your VWC Mechanicsville Team is on the MOVE!
Your VWC Mechanicsville care team is on the move to better care for you! We’re in the final stages of construction of a new 44,000-square-foot building. On Monday, March 25th,… Read More
We’re thrilled to announce the expansion of our Registered Dietitian Nutritionist (RDN) services!
When it comes to women’s health, we believe in a holistic approach encompassing mind, body, and spirit! Virginia Women’s Center is thrilled to announce the expansion of our Registered Dietitian… Read More
Ensuring Your Best Pregnancy Workshop
Each month, Fettle & Fit’s Dr. Casey Smith, PT, DPT, WCS, PCES, CSCS, will lead the Ensuring Your Best Pregnancy Workshop at the Short Pump location of Virginia Women’s Center…. Read More
Follow Us
[powr-social-feed id=2845eaf9_1542290999]
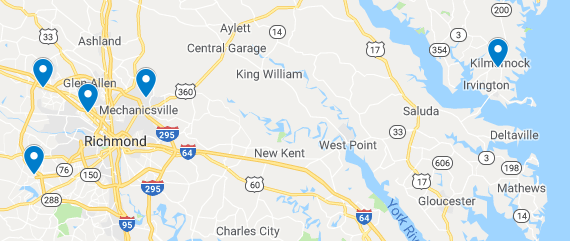
We recognize that in today’s busy world, convenience is paramount to you getting the care you need and deserve. And, that sometimes it’s easier to schedule your appointments near where you work, but you might want to deliver your baby or have surgery at a hospital near your home, Or, you simply have a hospital or location preference. That’s why our offices are strategically located to ensure that we’re convenient across the RVA region. And, we’re poised to deliver babies and perform gynecologic services at the hospital you want, including Bon Secours St. Mary’s Hospital, Memorial Regional Medical Center, St. Francis Medical Center, and HCA Henrico Doctors’ Hospital. Comprehensive, connected, collaborative care that’s convenient to you. And, don’t forget, we offer Saturday hours, too!
“Love, love, love this practice!”
“So delighted that I could have my Mammogram, Bone Density and yearly OBGyn check up all at the same location AND one right after the other! Thank You!”
“This is by far the most professional and courteous group I have dealt with in a long time. Everyone from check-in to checkout provided exemplary service.”
“If you are looking for a new woman's doctor or haven't been to one in awhile. Please please please try out this place!”
“So delighted that I could have my Mammogram, Bone Density and yearly OBGyn check up all at the same location AND one right after the other! Thank You!”
As a working mother of 2, it's often a challenge to find time to schedule routine exams for me. When Dr. Roberts recommended extra screening for breast cancer due to my family history, I was relieved to see I could have my mammogram right at VWC.. The scheduling and exam were both quick and easy. I feel great knowing I am being proactive with my own health!
Our Offices
Mechanicsville Please note new address
Address:
Contact:
Hours:
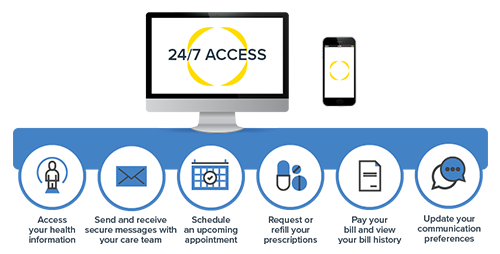
Patient Portal
Access your personal health information and test results, securely message your care team, and manage payments, all from one spot and at your convenience, 24/7.
- See your provider's openings and schedule appointments.
- Review prescription medications and request renewals.
- View payment history and pay current invoices.

About Privia Women’s Health
We’re proud members of Privia Women’s Health where we give caring for women the prominence it deserves.
We take a thoroughly modern and holistic approach to women’s health. We know what women, and their bodies, have to go through and we make sure every woman gets the individual care she needs.
Privia Women’s Health makes it easy for you to make your health a priority. We provide some of the latest advances in medicine, including innovative technologies, personalized care and a patient-centered approach to make sure every woman receives the individual care she needs.
Your health is your journey and no matter where you are, we’re here to help.